Theme: Impact of advanced therapies and technologies on pharmacovigilance practice and risk management
Pharmacovigilance Congress 2017
ConferenceSeries Ltd organizing gratifying Pharmaceutical conferences, welcomes you to attend the 10th Pharmacovigilance Congress to be held during September 20-21, 2017 in Charlotte, North Carolina, USA focuses on the advancements in pharmacovigilance and risk management.
The field of Pharmacovigilance is growing rapidly and its development is making tremendous impacts in medical sciences and pharmaceuticals. Pharmacovigilance Congress emphasizes on how the importance and significance can be gauged by the fact that it has made huge advancements over the course of time and is continuing to influence various sectors. .
Why to attend???
With members from around the world focused on learning about Pharmacovigilance and its advances; this is your best opportunity to reach the largest assemblage of participants from the Pharmacovigilance community. Conduct presentations, distribute information, meet with current and potential scientists, make a splash with new drug developments, and receive name recognition at this 3-day event. World-renowned speakers, the most recent techniques, developments, and the newest updates in Pharmacovigilance are hallmarks of this conference.
Target Audience:
- Pharmacovigilance Students, Scientists
- Pharmacovigilance Researchers
- Pharmacovigilance Faculty
- Medical Colleges
- Pharmacovigilance Associations and Societies
- Business Entrepreneurs
- Training Institutes
- Software developing companies
- Manufacturing Medical Devices Companies
- Data Management Companies.
ConferenceSeries Ltd organizing gratifying Pharmaceutical conferences welcomes you to attend the 10th Pharmacovigilance Congress to be held during September 20-21, 2017 in Charlotte, USA focuses on the advancements in Pharmacovigilance, Drug Safety and Risk management.
The field of Pharmacovigilance is growing rapidly and its development is making immense impacts in medical sciences and pharmaceuticals. Pharmacovigilance Congress accentuates on how the importance and significance can be gauged by the fact that it has made huge advancements over the course of time and is continuing to influence various sectors. Main theme of 10th Pharmacovigilance Congress is “Ensuring Drug Safety in Healthcare System”.
Track-1: Drug Safety:
Drug Safety (Pharmacovigilance) is the Pharmacological science relating to the collection, detection, determination, control, and inhibiting of adverse effects with Pharmaceutical products. In this Track it is discussed about the Drug Safety and its applications in various fields.
Track-2: Adverse Drug Reactions:
An Adverse Drug Reaction (ADR) is a response to drug which is noxious and unintended and which occurs at doses normally used in man for the prophylaxis, analysis, or therapy of disease or for the modification of physiological function. Many Adverse Drug Reactions represent an excess of the drug's therapeutic effects Manufacturers, professionals, and consumers should report Post Marketing Reports on adverse effects. How we reduce the adverse drug reactions is discussed in Reduction of adverse drug reaction by Nanotechnology topic.
Track 3: Pharmacovigilance and Risk Management:
Pharmacovigilance and Risk Management plays major role in Drug industry. The new turn in Drug industry is to use Information technology in pharmacovigilance companies. Drug Industry need to promoting companies in pharmacovigilance practice and the Review of software’s used in pharmacovigilance and clinical trials. Monitoring unlicensed, off labels and orphan drugs is major task in Risk Management. In this conferences so many experts from different Pharmacovigilance CRO’s, Pharmacovigilance service providers were participates and shares their knowledge and discusses about the new updates.
Track 4: Continental Pharmacovigilance:
Continental Pharmacovigilance gives an extension to Ensuring compliance with local and international requirements. To operate more efficaciously across multiple regions
Track 5: Good Pharmacovigilance Practice:
Pharmacovigilance and Pharmacoepidemiology mainly deal to avoid the insufficient evidence of safety from clinical trials. To maintain the reporting practices avoid major problems in risk management. In addition, it is important to intensify on Signal investigation through observational studies and Interpreting safety signals. The pharmacovigilance Clinical Trials services companies must have the Pharmacovigilance Certification.
Track 6: Pharmacovigilance Significance &Scope:
Pharmacovigilance and its Significance and Scope present the case for the importance of pharmacovigilance, to record its growth and potential as an important discipline within Medical science, and to describe its impact on patient welfare and public health and to know what is pharmacovigilance. In this track, we discussed about the Significance of pharmacovigilance. We also discussed on Pharmacovigilance role in healthcare system. Worldwide every year so many pharmacovigilance conferences were going on.
Track 7: Pharmacokinetics and Pharmacodynamics:
Drugs may undergo, Pharmacokinetics and Pharmacodynamics and Toxicity testing through animal testing. This data allows researchers to allometrically estimate a safe starting dose of the drug for clinical trials in humans. In this track mainly we focused on Advances in pharmacodynamics interactions, Drug and substance abuse, Drug-Drug Interactions. Also discussed about the Pharmacy Practices to maintain the Pharmacokinetics and Pharmacodynamics and its Challenges and its guidelines. There are several Challenges in compounding and dispensing practice in Pharmacokinetics and Pharmacodynamics.
Track 8: Pre-Clinical and Clinical Trials:
The main goals of Pre-Clinical and Clinical Trials are to determine the safe dose and start to assess product's safety profile. In Designing of trials, there are pre-clinical studies and different phases of clinical trials. In this trails they estimate the Bioassay and its types. The Data collection and quality control is the major part in Pre-Clinical and Clinical Trials. For some specific drugs, there are some Alternative trials designs and models are used. Pre-Clinical and Clinical Trials are conducted as Multi center trials and monitoring basis. Each Pre-Clinical and Clinical Trials study of a drug, biological product, or medical device regulated by the FDA must be reviewed, approved, and monitored by the Regulatory authorities and ethics committee. There are several types of clinical trials like Clinical Trials on drugs used in respiratory disorders. Advanced Information technology in clinical trials improves the quality of Pre-Clinical and Clinical Trials.
Track 9: Clinical Trials on Various Disorders:
Clinical Trials on Drugs used in Various Disorders, Pharmacotherapy and Pharmacotherapeutics Emerging technology in clinical trials track mainly deals on the clinical trials in long chronic diseases and several disorders. In This track we discussed about the Recent clinical trials on AIDS, Clinical trials on benign and malignant tumours, Clinical trials on cardiovascular diseases, Clinical trials on diabetes, Clinical Trials of monoclonal and polyclonal antibodies, Clinical trials on drugs used in psychological disorders The current research mainly focus on Applications of Biomarkers in clinical trials.
Track 10: Clinical Research and Statistics:
In Clinical Research Statistics plays major role. Depends upon the Statistics the clinical trials go for regulatory submissions. There are several guidelines mainly ICH guidelines for clinical research and its statistics follow for the clinical research. Stastical analysis of past pharmacovigilance and Adverse Drug Reactions reports also consider for the regulatory submission.
Track 11: Case Report in Clinical Trials:
Case Report in Clinical Trials plays major role in clinical research. In this track we discussed about the several case reports like Cancer case reports, Cardiovascular trials case report, Case studies on sexually transmitted diseases, Case studies on type 1 and type 2 diabetes, Case reports on drugs used in pregnancy and lactation.
Track 12: Biopharmaceutics:
In Clinical Pharmacology and Bio pharmaceutics track we discussed about the Rational drug management of cancer, diabetes and cardiovascular disorders, and Management of psychiatric disorders and autoimmune disorders. Along with clinical trials Bioavailability and Bioequivalence studies also plays major role in clinical research.
Track 13: PV Data Base Management:
There is advantage in centralising all safety data, clinical data, analysis and reporting with one provider. Pharmacovigilance Software tool provides comprehensive analysis of adverse events arising from the use of pharmaceutical products (Medicinal Product, Medical Device, Vaccines, Non-Drug Therapy and Veterinary Medicinal Product). The Drug Safety database allows the risk- benefit analysis of medicinal products taking into account new and emerging information, in the context of cumulative information. Pharmacovigilance since beginning has been a compliance driven activity, wherein your regulatory compliance determines company’s Risk Assessment scores. A Drug Safety Database offers scheduling of alerts for expedited cases, follow-up cases and PSUR/PADER reports submission to meet regulatory timeline compliance.
Track 14: PV Consulting’s and Business Opportunity:
Due to the changing resources necessary to fulfil the regulatory requirements, some companies also choose to outsource or out task Regulatory Affairs to external service providers. Regulatory Affairs department is constantly evolving and growing and is the one which is least impacted during the Acquisition and Merger, and also during recession. The stringent regulations on safety monitoring and their periodical revision have led to increased Safety Data collection, analysis and regulatory surveillance, and increased costs. Thus, Pharmacovigilance has become a critical phase in clinical development programs.
Track 15: Regulatory Affairs:
Regulatory affairs for clinical trials is the major part in the clinical trials approaches. Every clinical trial must be conduct according to the Regulatory affairs guidelines. There are several Regulatory affairs depending upon the countries. Regulatory Affairs departments are growing within companies. Global harmonization in standards has led to consistent approach in regulatory submissions and hence its review.
Track 16: Growth strategies in Pharma:
Strategies for Growth in Pharma Environment track mainly focused in Strategic development towards FDA approval and Post market product surveillances. The updates and advances in Pharmacovigilance regulation system are discussed in Advances in changing pharmacovigilance regulation system track.
Track 17: Pharmacy Practices and its Challenges:
In Pharmacy Practices and its Challenges track mainly focused on Pharmacy practice and its guidelines and Challenges in compounding and dispensing practice. Dosage regimen, Drug Toxicity and drug safety measures place important position in clinical research.
The Significant need of pharmacovigilance Leading by Exaggerating regulatory compliance, efficient maintenance of product life cycle and the growing need of prompt patient reporting. Pharmaceutical companies are increasingly entering into long term Agreements with CROs and BPOs for performing pharmacovigilance activities initiating from drug discovery phase to post market surveillance.The Following report Explores the global pharmacovigilance market based on clinical trial phases, reporting, service and geography Methods. In terms of clinical trial phases this market is studied for pharmacovigilance in preclinical studies, phase I trial, phase II trial, phase III trial, and phase IV. Spontaneous reporting, intensified ADR reporting, targeted spontaneous reporting, cohort event monitoring, EHR mining are the reporting methods analyzed in this report. Besides, In-House pharmacovigilance and contract outsourcing are the pharmacovigilance services considered in this Analysis. global pharmacovigilance market Collection of North America, Europe, Asia-Pacific, Middle East and North Africa, and Latin America are the regional markets. This Analysis also includes country level analysis for the national markets that hold major hold in the respective regional markets.
This Market Analysis Report Considering the 2015 as the base year and providing Market estimations and forecast for the period 2014 to 2022 for all the mentioned segments along with their respective Compounded Annual Growth Rate (CAGR %) for the period 2016-2022.This Analysis Providing Quantitative as well as Qualitative market assessment information such as market dynamics: drivers, challenges and future prospects. This Analysis includes competition assessment tools known as fractal map analysis for analyzing the market competition and attractive investment proposition . The Whole report here Concluding with Highlighting the company profile key information of major participants operating in global pharmacovigilance market.
pharmaceutical companies adopted the In-house pharmacovigilance and contract outsourcing as major Reporting method services in pharmacovigilance. The process is often being outsourced to contract outsourcing companies and it is anticipated that the same trend will continue through the forecast period. Contract outsourcing segment takes over one half of the market share and is anticipated to progress at a faster CAGR than in-house pharmacovigilance. Contract outsourcing is observed to be a faster and cost effective option; the CROs resourced are valued in the market for their core expertise in the respective processes. Furthermore, featured advantages such as reduction in overhead expenditures, focusing on core competencies, and workforce streamlining also has been urging pharmaceutical companies to opt for contract outsourcing. In-house pharmacovigilance system will still remain in practice majorly opted by large pharmaceutical companies for their high profile drugs and by small players for cost containment purposes.
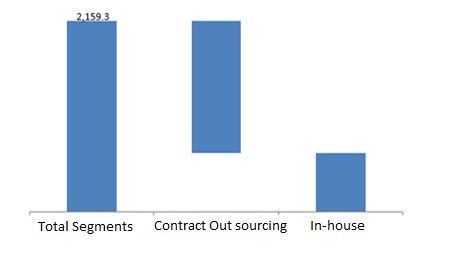
Preclinical studies, phase I, phase II, phase III, phase IV trials or post marketing surveillance are the drug development phases that cumulatively make the global pharmacovigilance market.
Among the mentioned segments, phase IV or post market reporting takes the largest market share in 2016. This stage is characterized by prompt collection of post market development, adverse events, and examination and monitoring of associated hazards. Growth in the number of novel drugs being introduced in the market has necessitated the need for post marled surveillance thus propelling phase IV pharmacovigilance segment. In addition, necessity for risk assessment studies during late stage drug development, decision on precise medication and establishment of safety data are the prime factors that constitute to the rapid market growth of phase III pharmacovigilance segment.
Top of Form
North America, Europe, Asia Pacific, Middle East and Africa, and Latin America together make for the global pharmacovigilance market.
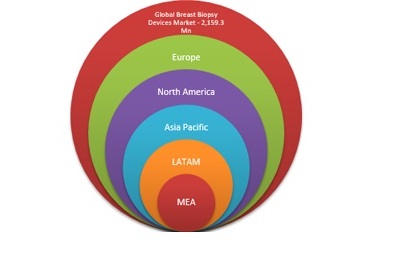
North America is the largest regional market for pharmacovigilance global processes. Dominance of North America is majorly attributed to growth in clinical research activities in the region. Due to the presence of a multitude of research units and pharmaceutical companies in the country made U.S. as the largest country level market for pharmacovigilance,. Regulatory framework demanding strict compliance from pharmaceutical companies in the U.S. is also a contributing factor to the large scale pharmacovigilance activities in the country. Asia-Pacific countries such as Japan, Korea, Australia and others are undergoing a rapid growth in pharmacovigilance market; outsourcing of clinical activities to south Asia plays a critical role in Asia-Pacific market.
The detailed classification of the global pharmacovigilance market is as follows:
§ North America (U.S. & Canada)
§ Europe (U.K., Germany and Rest of Europe)
§ Asia-Pacific (Japan, China and Rest of Asia-Pacific)
§ Middle East and Africa
§ Latin America
The global pharmacovigilance and drug safety software market is poised to grow at a CAGR of 6.5% during 2016-2019, and is expected to reach a value of $154.1 Million in 2019.In this Analysis, the global pharmacovigilance and drug safety software market has been classified into many segments on the basis of functionality, namely, adverse event reporting software, drug safety audits software, issue tracking software, and fully integrated software. The global pharmacovigilance and drug safety software market is divided into two segments on the basis of delivery mode, namely, on-premise delivery mode and on-demand/cloud based (SaaS) delivery mode. The global pharmacovigilance and drug safety software market is also segmented on the basis of end users including pharma and biotech companies, contract research organizations (CROs), business process outsourcing (BPO) firms, and other pharmacovigilance service providers.
Factors such as rising incidence rates of adverse drug reactions (ADRs) and increasing adoption of pharmacovigilance software by outsourcing companies are driving the growth of the global pharmacovigilance and drug safety software market. In addition; government regulatory bodies (such as the U.S. FDA and EMEA) have intensified safety regulations for prior and post commercialization of drugs, which has increased pressure on the pharmaceutical and biotechnology companies to manufacture safe drugs and evaluate their results post sales. Thus, growing complexity related to drug safety regulations is expected to drive the growth of the market during the forecast period.
As of 2014, North America holds the largest share of the global pharmacovigilance and drug safety software market, followed by Europe. However, Asian and Latin American countries represent high growth markets, owing to a rise in research outsourcing by pharmaceutical giants and growing public and private investments in pharmaceutical R&D in these emerging nations.
· ArisGlobal (U.S.)
· Ennov Solutions Inc. (U.S.)
· EXTEDO GmbH (U.S.)
· Online Business Applications, Inc. (U.S.)
· Oracle Corporation (U.S.)
· Sarjen Systems Pvt. Ltd (India)
· Sparta Systems, Inc. (U.S.) and
· United BioSource Corporation (U.S.)
are some of the key players in the global pharmacovigilance and drug safety software market applications, growing need for improved data standardization, rising prevalence of chronic diseases, increasing pressure to curb healthcare spending, and growing need for improved patient outcomes.
On the basis of type, the market is segmented into descriptive, predictive, and prescriptive analytics. The descriptive analytics segment is expected to account for the largest market share in 2016. This can be attributed to the significant usage of descriptive analytics by life science stakeholders to gain a better understanding of the past trends and event occurring in real-time.
On the basis of application, the life science analytics market is segmented into research and development (clinical trials and preclinical trials), sales and marketing support, regulatory compliance, supply chain analytics, and pharmacovigilance. The sales and marketing support segment is expected to grow at the highest CAGR during the forecast period. The high growth of this segment can be attributed to the increasing adoption of analytics by life science companies to align their sales and marketing campaigns. The growing importance of post-marketing surveillance is also boosting the utilization of analytics for sales and marketing.
Conference Highlights
- Drug Safety
- Adverse Drug Reactions
- Clinical Data Management
- Case Study
- Good Pharmacovigilance Practice
- Pharmacovigilance Risk management plans and new risk- benefit analysis tools
- Pharmacokinetics and Pharmacodynamics
- Overview of Clinical Trials and Post Marketing
- Preclinical and Clinical Trials on Various Disorders
- Case Report in Clinical Trials
- Pharmacovigilance on Biopharmaceutics
- Â PV Data Base Managemaent
- PV Consultings And Bussiness Opportunity
- Regulatory Affairs
- Growth Strategies in Pharma
- Pharmacy Practices and its Challenges
- Entrepreneurs Investment Meet
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | September 20-21, 2017 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | ||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Pharmacovigilance
- Journal of Clinical & Experimental Pharmacology
- Journal of Clinical Trials
Abstracts will be provided with Digital Object Identifier by










































































